
Introduction
Learn how to make litmus paper with this step by step guide!
Welcome! In this guide, we will be teaching you how to make your very own litmus paper and do other experiments. It's fun and easy to make, and it can be made using household ingredients. Children would love to make this, and it really looks magical.
What Is Litmus Paper?
Litmus paper is an indicator used to test whether a substance is acidic or basic. You simply dip the paper in your substance, and it will change colour depending on whether it is acidic or basic. It will go red in an acid and blue in a base. It is a lot of fun to make and is very easy. The kids would love to get stuck in with this project!
Materials Needed
- Blotting Paper, Cut into strips
- 2 cups Red Cabbage, Chopped
- 10ml Lemon Juice
- 10ml Vinegar
- Soap
Instructions
- Boil your red cabbage until the water it is boiling in is a red-purple colour from the cabbage. Strain the red cabbage from the solution.
- Cut your blotting paper into small strips.
- Put your blotting paper strips into the solution and leave them there to soak.
- Take them out and leave to dry.
- Your litmus strips are now ready to use!
Where to Buy Blotting Paper
Update:
An increasing number of people have told me that they couldn't try this experiment out as they couldn't find any blotting paper in the shops. To all of you out there, the very same happened to me and I'm sorry I didn't address this issue earlier. If you cannot find any blotting paper in the shops, you can buy blotting paper on amazon

Experiment One
Experiment Time!
Now that you have your litmus paper strips, it is now time to experiment.
- Dip a strip of litmus paper into the substance.
- If it turns red, that substance is an acid.
- If it turns blue, that substance is a base.
- If it stays the same, that substance is neutral.
Example:
Let's take lemon juice for example. Dip a litmus paper strip into the lemon juice. It will turn red as the lemon juice is acidic.
- Try all the substances below.They will give you some ideas on what to test. You can print the table out and tick off whether each substance is acidic, basic or neutral.
Use Your Litmus Paper to Test These Substances
Substance
|
Acidic?
|
Basic?
|
Neutral?
|
|---|---|---|---|
Vinegar
| |||
Milk of Magnesia
| |||
Washing Up Liquid
| |||
Fizzy Drinks
| |||
Sour milk
| |||
Toothpaste
| |||
Soapy Water
| |||
Lemon Juice
| |||
Baking Soda Solution
| |||
Black Coffee
| |||
Tomato Juice
| |||
Orange Juice
|

Testing Rainwater
Rainwater is generally slightly acidic. This occurs from acid rain. The carbon dioxide that is pumped from the factories goes out into the air and mingles with the clouds. This causes carbonic acid. Acid rain is very damaging. It can damage limestone monuments and kill fish in the rivers. You can test whether the rainwater in your area is slightly acidic. Here's how:
- Make a rain gauge out of a bottle or glass jar. Place it outside away from any trees or shelter that may prevent the rain to go in.
- Place a funnel over your rain gauge.
- Leave the rain to fill it.
- Dip your strips of litmus into the rainwater.

How Does It Work?
The red cabbage you used can be used as pH indicator. It is red or pink in acids (pH < 7), purple in neutral solutions and ranges from blue to green to yellow in alkaline solutions (pH > 7).
The pH Scale
Knowing whether a substance is acidic or basic is only the first step. Scientists in the laboratory need to know how strong/weak an acid or base really is. Sodium Hydroxide is a very strong base and can burn your skin if you come in contact with it. Therefore, there is a scale called the pH scale which ranges from 1-14.
- Very strong Acids are around 1-2 on the pH Scale.
- Weaker acids are 3-6 on the pH scale.
- Neutral (neither acidic or basic) are 7
- Weak bases are 8-9 on the pH scale
- Very strong bases are 10-12 on the pH scale
To determine how strong/weak an acid or base is and where it is on the pH scale, scientists use universal indicator paper which can be seen on the top right. You dip the paper into the substance of your choice and it will change colour. You can then determine how strong an acid or base is by judging the colour of the paper to the colours on the pH scale. See picture at top.
Experiment Two
Neutralisation
There are ways to make a substance neutral (having a pH the same as water). Neutralisation is done when the same amount of an acid is added to a base.
Real-Life Applications:
There are many real life applications to this concept. Let's say that a truck containing a strong acid overturned and the acid spilled everywhere. It would destroy and burn everything. The emergency team will add a base such as lime to the acid. The acid will be neutralised making it virtually harmless. Or let's say you had indigestion. Indigestion is caused by strong acids in your stomach. You might take indigestion tablets to relieve the pain.The indigestion tablets are bases. So, these bases neutralise the acids making them virtually harmless.
Instructions
- Add a teaspoon of washing-up liquid into a cup or container.
- Add the same amount of vinegar into the washing-up liquid.
- This way the two substances become neutralised.
- Dip a strip of litmus paper into it. If the strip stays the same colour, it is proven that the two substances have been neutralised. A great way of showing this concept in action.
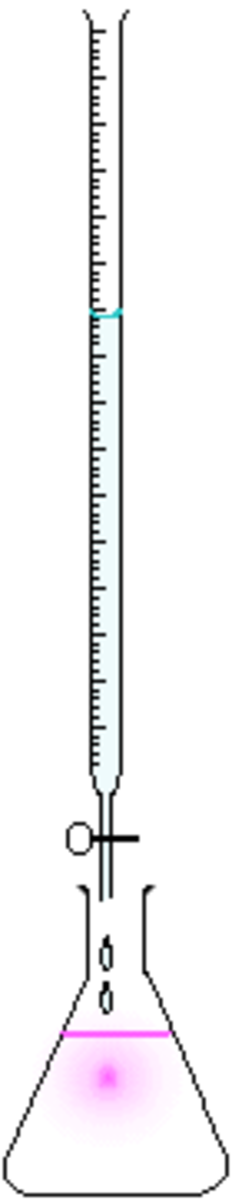
Acid-Base Titrations
- The topic of neutralisation leads onto another topic. When you neutralise an exact amount of acid with a base, a salt + water is formed. This is known as an acid-base titration.
- The acid goes on top, and the base goes on the bottom.
- An indicator such as methyl orange or litmus is put into the base. It turns yellow. Then the acid is put into the base drop by drop until the methyl orange indicator turns orange.
- When the indicator turns orange, you can be sure that the two substances are neutralised. The solution is poured into an evaporating dish and is evaporated off.
- It is noticed that a salt is left.




No comments:
Post a Comment
Due to the high number of spammy comments we have decided to initiate comment moderation so that we can maintain our quality standards and make good environment for our visitors. Please leave your comment